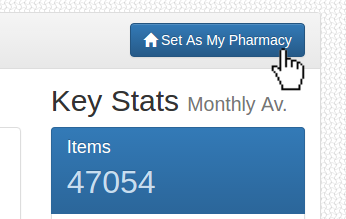
Pharmacy Not Set
Please use the search function to find your pharmacy, then click 'Set As My Pharmacy'. This can also be modified in the Settings page.

This site is intended for Healthcare Professionals only
2022-11-16 11:26:17 (3 year(s) ago)
Martindale Pharma has made the MHRA aware that the GTIN in the 2D barcode and the printed variable data represents the branded version of the product (Venlalic® XL 300 mg prolonged-release tablets).